Highlight
Monte Carlo Simulation Study of Cavitation in Metastable Fluids
Achievement/Results
Christopher Rasmussen – Trainee; Alexander V. Neimark – IGERT Adviser (Rutgers University); Aleksey Vishnyakov – Collaborator (Rutgers University); Mattias Thommes – Industry Collaborator (Quantachrome Corporation, Boynton Beach, Florida); Freddy Kleitz – International Collaborator (Université Laval, Québec, Canada); Tim Lebo – Undergraduate Assistant (Rutgers University)
Monte Carlo Simulation Study of Cavitation in Metastable Fluids
Cavitation is the phenomenon of spontaneous formation of bubbles in metastable fluids. Cavitating bubbles are entirely unstable and tend to collapse producing a shock wave. Cavitation is widespread in nature and industry; it has multiple physiological consequences, such as decompression after diving, and biomedical applications. Cavitation of nitrogen is used to fragment kidney and gall bladder stones, and has been recently suggested as a means to unintrusively rupture cancer cells and for targeted drug delivery. Due to a spontaneous nature of cavitation, it is impossible to study the process of bubble formation in macroscopic systems.
This work aims at a better understanding of fundamentals of cavitation by studying the bubble nucleation in metastable fluids confined to nanoscale pores. Nanoconfinement reduces thermal fluctuations in metastable fluids that allows us to explore the very onset of the cavitation in great details. The goal is to predict the rate of nucleation as a function of the degree of fluid metastability in pores of different sizes and to up-scale the results to macroscopic environments. We apply the Monte Carlo simulation in conjunction with experimental studies on reference nanoporous materials. Cavitation of a metastable adsorbate in blocked mesopores causes a sharp step on the desorption isotherm near the closure point of the hysteresis loop 1. We hypothesize that the position of cavitation depends on the free energy barrier of formation of the critical nucleus.
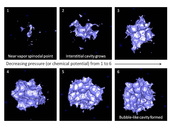
Recently, new experimental work with highly-ordered mesoporous materials has shed light on the behavior of phase change in nanosized structures 2. We examined the adsorption/capillary condensation and desorption/cavitation of nitrogen at its boiling point (77.4K) onto the highly-order porous network of SBA-16, a mesoporous silica with a cubic Im3m structure of spheroidal pores. To calculate the simulated isotherm, we employed Grand Canonical Monte Carlo (GCMC) and Gauge Cell Monte Carlo methods. The GCMC scheme mimics the experimental conditions of cavitation as a spontaneous process. The Gauge Cell method was designed to simulate the metastable and labile states, not observable in experimental work [3,4]. Characteristic configurations of growing nanoscale bubbles are shown in Figure 1 5. The systems modeled are spherical silica pores, ranging from 5.7nm to 9.7nm in diameter. We achieved an excellent agreement between the simulated and experimental data on nitrogen adsorption-desorption isotherms showing the cavitation phenomenon (Figure 2). The thermodynamic integration along the simulated isotherm shown in Figure 1 gives the energy barrier of nucleation, which determines the rate of bubble formation. We compare the calculated nucleation energy of each system studied and its equivalent experimental cavitation pressure. (Figure 3). Our results suggest that the cavitation in nanopores occurs around at 40-50 kT, regardless of the pore size. This conclusion implies that there is a limit to the influence of the porous structure on the onset of cavitation, and thus, cavitation in wide nanopores may be employed to model cavitation in macroscopic systems.
The impact of this work is broad. There is much recent interest on the role of cavitation for drug delivery and non-invasive treatment of tumors, including some work using a porous medium as a nucleation site. There are immediate applications in porous material characterization. This fundamental work can help understand the physics of cavitation and thus influence the design and use of novel biomedical applications. Future work on this topic includes refinement of simulations and calculations, and observing the effect of temperature and different adsorbates on cavitation. We will continue to foster our collaborations with universities and companies around the world. This work is being presented at The 8th International Symposium on the Characterization of Porous Solids, which will be held in Edinburgh in June 2008.
1 P. I. Ravikovitch and A. V. Neimark, Langmuir, 2002, 18, 9830-9837. 2 M. Thommes, B. Smarsly, P.I. Pavikovitch, Langmuir, 2006, 22, 756-764. 3 A. V. Neimark and A. Vishnyakov, Journal of Chemical Physics, 2005, 122, 234108. 4 A. V. Neimark and A. Vishnyakov, Journal of Physical Chemistry, 2006, 110, 9403-9412. 5 A. V. Neimark and A. Vishnyakov, Journal of Chemical Physics, 2005, 122, 054707.
Address Goals
All of the work presented above addresses the NSF strategic goal of DISCOVERY. We have suggested and validated a novel approach to studies of cavitation in metastable fluid. The success of this program will lead to the establishment of a new experimental technique, guided by molecular simulations, which will allow to study the spontaneous processes of cavitation with a high precision. Currently, the experimental studies are limited to macroscopic systems, such as overheated droplets of millimeter size. In such large systems, the fluctuations are not constrained: spontaneous boiling occurs quickly, at the degree of metastability characterized by the nucleation barrier of ~70 kT. Our idea to use nanoporous confinements to reduce the fluctuations and metastable states allows us to study experimentally the systems with nucleation barriers of ~50 kT, which are inherently unstable on the macroscopic scale. At the same time, the Monte Carlo simulations with the original gauge cell method that we developed are capable of modeling the whole range metastable and labile states bridging the gap between the nanoscale and macroscopic systems. Based on the combination of simulations and experiments with nanoporous confinements, we suggested a new approach to the assessment of the metastable system stability and the rate of cavitation.
The fundamental nature of this work is well suited to the ideals of the IGERT program of interdisciplinary studies, which involve industrial and international collaborations. Understanding a phenomenon as widespread as cavitation has important practical applications in physiology and medicine. There is much recent interest in the cavitation-based techniques for drug delivery and non-invasive treatment of tumors, including some work using a porous medium as a nucleation site. There are immediate applications of our results in the porous material characterization. This fundamental work can help understand the physics of cavitation and thus influence the design and use of novel biomedical applications.
The secondary NSF strategic goal of this work is LEARNING. The project serves as an excellent introduction into the world of molecular simulations for the IGERT students. Skills gained in the simulation of these systems are immediately applicable in future IGERT projects, not limited to the study of cavitation phenomena. The students acquire multidisciplinary skills not only in the statistical mechanics and theoretical physical chemistry, but also in molecular simulations and adsorption experiments. The graduate and undergraduate students, involved in this project, specifically benefit from the international and industrial collaborations. The scientific results will be included into the IGERT graduate course on Nanoscale Thermodynamics and Transport (Neimark).